DATA
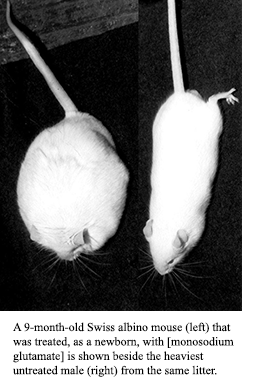
MSG and Obesity
The human body is a wonderful thing. There are systems working together that create checks and balances to keep us well - or to fight disease should we encounter it. Obesity can happen when one or more of those systems is not working. It can happen when stress or depression throw the endocrine system off balance, and there is loss of appetite control. It can happen when there is an injury (a blow to the head for example), that disrupts the endocrine system. And it can happen when MSG or any other ingredient that contains manufactured free glutamate, the toxic component in monosodium glutamate (MSG), is fed to the unborn and the very young - fed to them before their blood brain barriers are well enough developed to protect the brain cells destined to control endocrine function later in life.There are actually two separate and possibly distinct paths through which glutamate-induced obesity can thrive. In the first, free glutamate moves through the incomplete (immature) blood-brain-barrier in the arcuate nucleus of the hypothalamus of the unborn and very young and kills brain cells (neurons), causing permanent damage to the endocrine system that controls appetite and satiety. In other words, when delivered to the fetus in utero and/or fed to the very young, free glutamate causes brain damage that destroys the neurons that would have regulated appetite and satiety had they not been obliterated by excitotoxic free glutamate.
Diet and exercise will do little of a permanent nature for a person whose endocrine system has been damaged in this way.
Second, in both children and adults, continuous ingestion of free glutamate will raise various hormone levels, including levels of the hormone insulin. When the insulin level is raised, the glucose level is lowered, which is the body's signal to eat more food in order to raise (balance) the glucose level. With the brain signaling the body to consume more food, diet and exercise have little chance of controlling weight gain without a permanent highly restrictive diet.
Data: MSG kills brain cells...
In 1957, Lucas and Newhouse (26) first noticed that severe retinal lesions could be produced in suckling mice (and to some extent in adult mice) by a single injection of monosodium glutamate. In the late 60s, Olney (59) became suspicious that obesity in mice, which was observed after neonatal mice were treated with monosodium glutamate for purposes of inducing and studying retinal pathology, might be associated with hypothalamic lesions caused by monosodium glutamate treatment; and in 1969 Olney first reported that monosodium glutamate treatment did indeed cause brain lesions, particularly acute neuronal necrosis in several regions of the developing brain of neonatal mice, and acute lesions in the brains of adult mice given 5 to 7 mg/g of glutamate subcutaneously (59).Research that followed confirmed that glutamate, which was routinely given as monosodium glutamate (brand name Accent), induces hypothalamic damage when given to immature animals after either subcutaneous (60,61,62,63,64,66,67,68,69,70,71,72,73,74,75,76,77,78, 81) or oral (67,73,74,76,82,83,84,85,86) doses.
Data: Brain cells damaged by MSG lead to endocrine disorders...
 Olney found not only hypothalamic lesions in 1969, but described stunted skeletal development, obesity, and female sterility, as well as a spate of observed pathological changes found in several brain regions associated with endocrine function in maturing mice which had been given monosodium glutamate as neonates (59).
Olney found not only hypothalamic lesions in 1969, but described stunted skeletal development, obesity, and female sterility, as well as a spate of observed pathological changes found in several brain regions associated with endocrine function in maturing mice which had been given monosodium glutamate as neonates (59).
Longitudinal studies in which neonatal/infant animals were given doses of monosodium glutamate and then observed over a period of time before being sacrificed for brain examination, repeatedly supported Olney's early findings of abnormal development, behavioral aberration, and neuroendocrine disorder. Animals treated with monosodium glutamate as neonates or in the first 12 days of life were shown to suffer neuroendocrine disturbances including obesity and stunting, abnormalities of the reproductive system, and underdevelopment of certain endocrine glands (59,68,70,86,88,89,90, 92,93,94,95,96,97,98,99,100,101,102,103,104,105,106) and possible learning deficits either immediately or in later life (92,95,96,107,108,109,110,112,113). In addition, there were reports of behavioral reactions including somnolence and seizures (114,115,116,117,119,120,121); tail automutilation (94,108); and learned taste aversion (110). Irritability to touch was interpreted as conspicuous emotional change by Nemeroff (94). Lynch (14) reported hyperglycemia along with growth suppression. He noted that hyperglycemia did not occur when subjects were given intact protein containing a large amount of glutamate.
Olney et al. (122,123,124) have written a number of review articles which summarize the data on neuroendocrine dysfunction following monosodium glutamate treatment. Nemeroff (125) has written another.
Relating animal studies to humans...
Findings of neurotoxicity and neuroendocrine dysfunction in laboratory animals raised questions about the effects that monosodium glutamate might have on humans. Since it would be unthinkable to administer doses of monosodium glutamate that might produce the same sorts of neurotoxicity and neuroendocrine dysfunction as found in laboratory animals, researchers had no alternative but to make decisions based on the best of the animal studies. "Best," in this case, would be studies that would most closely parallel the true human condition.At the time, a seemingly logical first step was to study the effects of monosodium glutamate on nonhuman primates; and, as noted elsewhere, hypothalamic lesions had been demonstrated in monkeys as early as 1969 (61). A seemingly logical second step was to study "normal" ingestion of monosodium glutamate as opposed to some kind of forced feeding. Many felt that ad libitum feeding of laboratory animals parallels the human situation more closely than either subcutaneous or gavage administration of monosodium glutamate, and that ad libitum feeding studies were, therefore, the vehicle of choice. Ad libitum feeding would give animals free access to feed or water thereby allowing the animal to self-regulate intake. Some tended to disagree, feeling that the ad libitum feeding studies were, by and large, studies that had the greatest potential for minimizing the amount of monosodium glutamate actually ingested while registering the irrelevant amount of monosodium glutamate available.
Two studies that demonstrate neurotoxic reactions after ad libitum feeding of monosodium glutamate are reported here. In a 1979 study done as part of a project designed to evaluate a developmental test battery for neurobehavioral toxicity in rats, in which rats were exposed to monosodium glutamate and other food additives mixed with ground Purina rat chow beginning five days after arrival at the laboratory (109), it was demonstrated that high doses of dietary monosodium glutamate produce behavioral variations. Monosodium glutamate was mixed with food as opposed to being administered subcutaneously or by gavage. A year later, dietary studies demonstrated that weanling mice will voluntarily ingest monosodium glutamate and that such voluntary ingestion results in readily detectable brain damage (127).
MSG is now a research tool used to produce obesity...
By the early 1980s, evidence of monosodium glutamate induced brain lesions and resultant endocrine disorders, including obesity, were so well documented that researchers interested in brain function and/or development of drugs with which to treat disease conditions in which glutamate played a role, began to use monosodium glutamate as an ablative or provocative tool with which to kill selected brain cells and induce obesity.Free glutamate is readily available to the unborn, neonates, and infants...
It is now recognized the fetus can receive free glutamate in utero through the mother's diet if the mother is consuming processed food that contains free glutamate and after birth, the infant can receive free glutamate through the mother's milk if the mother is consuming processed food that contains free glutamate, and/or the infant can receive free glutamate in infant formula. Free glutamate is invariably present in infant formula. The greatest amounts of free glutamate in infant formula will be found in hypoallergenic formulas--which are typically soy based.The newborn and young children are also introduced to free glutamate through vaccines. All live virus vaccines, and some, if not all of the others, contain free glutamate. The free glutamate in vaccines will typically be contained in one or more ingredients that give no clue to the fact that they contain free glutamate.
Studies have demonstrated that free glutamate can cross the placenta during pregnancy (1-2), can cross the blood brain barrier in an unregulated manner during development, and can pass through the five circumventricular organs, which are "leaky" at best at any stage of life (3-5). Moreover, the blood brain barrier is easily damaged by fever, stroke, trauma to the head, seizures, ingestion of processed free glutamic acid, and the normal process of aging (5-6). It is generally accepted that the young are particularly at risk from ingestion of free glutamate. The blood brain barrier, once thought to prevent glutamate from entering the brain, is not fully developed until puberty.
Free glutamate has an effect on at least some hormone levels...
The extent of free glutamate's effect(s) on hormone levels is as yet unknown. It is known, however, that continuous ingestion of free glutamate raises some hormone levels, including the hormone insulin. When you raise the insulin level, you lower the glucose level, which is the body's signal to eat more food in order to raise (balance) the glucose level.There is evidence (from the FDA's own laboratory) that free glutamate in the general human diet can disrupt normal metabolism and affect insulin function. Lynch reported hyperglycemia along with growth suppression. He noted that hyperglycemia did not occur when subjects were given intact protein containing a large amount of glutamate (7).
A 2008 study by He et al. demonstrated that in a rural area of China, persons who ingested more monosodium glutamate were heavier than persons who ingested less monosodium glutamate. In the journal Obesity, it was reported that monosodium glutamate used in food may be associated with increased risk of obesity independent of physical activity and total food intake (8). The study is available online.
In 2000, Macho, Fickova, and Jezova found that early postnatal administration of free glutamate exerts an important effect on glucose metabolism and insulin action in adipocytes of adult animals (9).
References
1. Frieder, B, and Grimm, VE. Prenatal monosodium glutamate (MSG) treatment given through the mother's diet causes behavioral deficits in rat offspring. Intern J Neurosci. 23:117-126,1984.2. Gao, J, Wu, J, Zhao, XN, Zhang, WN, Zhang, YY, Zhang, ZX. [Transplacental neurotoxic effects of monosodium glutamate on structures and functions of specific brain areas of filial mice.] Sheng Li Hsueh Pao Acta Physiologica Sinica. 46:44-51,1994.
3. Price MT, Olney JW, Lowry OH, Buchsbaum S. Uptake of exogenous glutamate and aspartate by circumventricular organs but not other regions of brain. J. Neurochem. 36:1774-1780,1981.
4. Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Exp Neurol. 120:245-263,1993.
5. Blaylock, RL. Excitotoxins: The Taste That Kills. Santa Fe, NM: Health Press; 1994.
6. Nemeroff, C.B., and Crisley, F.D. Monosodium L-glutamate induced convulsions: temporary alteration in blood-brain barrier permeability to plasma proteins. Environ Physiol Biochem 5: 389-395, 1975.
7. Lynch JF Jr., Lewis LM, Adkins JS. [Division of Nutrition, FDA, Washington, D.C. 20204]. Monosodium glutamate-induced hyperglycemia in weanling rats. J S Fed Proc. 31:1477,1971.
8. He K, Zhao L, Daviglus ML, Dyer AR, Van Horn L, Garside D, Zhu L, Guo D, Wu Y, Zhou B, Stamler J; INTERMAP Cooperative Research Group. Association of monosodium glutamate intake with overweight in Chinese adults: the INTERMAP Study. Obesity (Silver Spring). 2008 Aug;16(8):1875-80.
9. Macho L, Fickova M, Jezova, Zorad S. Late effects of postnatal administration of monosodium glutamate on insulin action in adult rats. Physiol Res 2000;49 Suppl 1:S79-85.
14. Lynch, JF Jr, Lewis LM, Adkins JS. Monosodium glutamate-induced hyperglycemia in weanling rats. Fed Proc. 1971;30(2):460Abs (Abstract #1477).
26. Lucas DR, Newhouse JP. The toxic effect of sodium-L-glutamate on the inner layers of the retina. AMA Arch Ophthalmol. 1957;58(2):193-201.
59. Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164(880):719-721.
60. Olney JW, Ho OL, Rhee V. Cytotoxic effects of acidic and sulphur containing amino acids on the infant mouse central nervous system. Exp Brain Res. 1971;14(1):61-76.
61. Olney JW, Sharpe LG. Brain lesions in an infant rhesus monkey treated with monosodium glutamate. Science. 1969;166(903):386-388.
62. Snapir N, Robinzon B, Perek M. Brain damage in the male domestic fowl treated with monosodium glutamate. Poult Sci. 1971;50(5):1511-1514.
63. Perez VJ, Olney JW. Accumulation of glutamic acid in the arcuate nucleus of the hypothalamus of the infant mouse following subcutaneous administration of monosodium glutamate. J Neurochem. 1972;19(7):1777-1782.
64. Arees EA, Mayer J. Monosodium glutamate-induced brain lesions: electron microscopic examination. Science. 1970;170(957):549-550.
66. Everly JL. Light microscopy examination of monosodium glutamate induced lesions in the brain of fetal and neonatal rats. Anat Rec. 1971;169(2):312.
67. Olney JW. Glutamate-induced neuronal necrosis in the infant mouse hypothalamus. J Neuropathol Exp Neurol. 1971;30(1):75-90.
68. Lamperti A, Blaha G. The effects of neonatally-administered monosodium glutamate on the reproductive system of adult hamsters. Biol Reprod 1976;14(3):362-369.
69. Takasaki Y. Studies on brain lesion by administration of monosodium L-glutamate to mice. I. Brain lesions in infant mice caused by administration of monosodium L-glutamate. Toxicology. 1978;9(4):293-305
70. Holzwarth-McBride MA, Hurst EM, Knigge KM. Monosodium glutamate induced lesions of the arcuate nucleus. I. Endocrine deficiency and ultrastructure of the median eminence. Anat Rec. 1976;186(2):185-196.
71. Holzwarth-McBride MA, Sladek JR, Knigge KM. Monosodium glutamate induced lesions of the arcuate nucleus. II Fluorescence histochemistry of catecholamines. Anat Rec. 1976;186(2):197-205.
72. Paull WK, Lechan R. The median eminence of mice with a MSG induced arcuate lesion. Anat Rec. 1974;180(3):436.
73. Burde RM, Schainker B, Kayes J. Acute effect of oral and subcutaneous administration of monosodium glutamate on the arcuate nucleus of the hypothalamus in mice and rats. Nature. 1971;233(5314):58-60.
74. Olney JW, Sharpe LG, Feigin RD. Glutamate-induced brain damage in infant primates. J Neuropathol Exp Neurol. 1972;31(3):464-488.
75. Abraham R, Doughtery W, Goldberg L, Coulston F. The response of the hypothalamus to high doses of monosodium glutamate in mice and monkeys: cytochemistry and ultrastructural study of lysosomal changes. Exp Mol Pathol.1971;15(1):43-60.
76. Burde RM, Schainker B, Kayes J. Monosodium glutamate: necrosis of hypothalamic neurons in infant rats and mice following either oral or subcutaneous administration. J Neuropathol Exp Neurol. 1972;31(1):181.
77. Robinzon B, Snapir N, Perek M. Age dependent sensitivity to monosodium glutamate inducing brain damage in the chicken. Poult Sci. 1974;53(4):1539-1542.
78. Tafelski TJ. Effects of monosodium glutamate on the neuroendocrine axis of the hamster. Anat Rec. 1976;184(3):543-544.
81. Olney JW, Rhee V, DeGubareff T. Neurotoxic effects of glutamate on mouse area postrema. Brain Res. 1977;120(1):151-157.
82. Olney JW, Ho OL. Brain damage in infant mice following oral intake of glutamate, aspartate or cystine. Nature. 1970;227:609-611.
83. Lemkey-Johnston N, Reynolds WA. Nature and extent of brain lesions in mice related to ingestion of monosodium glutamate: a light and electron microscope study. J Neuropath Exp Neurol. 1974;33(1):74-97.
84. Takasaki, Y. Protective effect of mono- and disaccharides on glutamate-induced brain damage in mice. Toxicol Lett. 1979;4(3): 205-210.
85. Takasaki, Y. Protective effect of arginine, leucine, and preinjection of insulin on glutamate neurotoxicity in mice. Toxicol Lett. 1980;5(1):39-44.
86. Lemkey-Johnston, N, Reynolds WA. Nature and extent of brain lesions in mice related to ingestion of monosodium glutamate: a light and electron microscope study. J Neuropath Exp Neurol. 1974;33(1):74-97.
87. Olney JW. Brain damage and oral intake of certain amino acids. In: Levi G, Battistin L, Lajtha A, eds.Transport Phenomena in the Nervous System: Physiological and Pathological Aspects. New York: Plenum Press; 1976.
88. Reynolds WA. Lemkey-Johnston N, Filer LJ Jr, Pitkin RM. Monosodium glutamate: absence of hypothalamic lesions after ingestion by newborn primates. Science. 1971;172(990):1342-1344.
89. Matsuyama S. Studies on experimental obesity in mice treated with MSG. Jap J Vet Sci. 1970;32:206.
90. Redding TW, Schally AV, Arimura A, Wakabayashi I. Effect of monosodium glutamate on some endocrine functions. Neuroendocrinology. 1971;8(3):245-255.
92. Araujo PE, Mayer J. Activity increase associated with obesity induced by monosodium glutamate in mice. Am J Physiol. 1973;225(4):764-765.
93. Nagasawa H, Yanai R, Kikuyama S. Irreversible inhibition of pituitary prolactin and growth hormone secretion and of mammary gland development in mice by monosodium glutamate administered neonatally. Acta Endocrinol. 1974;75(2):249-259.
94. Nemeroff CB, Grant LD, Bissette G, Ervin GN, Harrell LE, Prange AJ Jr. Growth, endocrinological and behavioral deficits after monosodium L-glutamate in the neonatal rat: Possible involvement of arcuate dopamine neuron damage. Psychoneuroendocrinology.1977;2(2):179-196.
95. Nemeroff CB, Konkol RJ, Bissette G, et al. Analysis of the disruption in hypothalamic-pituitary regulation in rats treated neonatally with monosodium glutamate (MSG): Evidence for the involvement of tuberoinfundibular cholinergic and dopaminergic systems in neuroendocrine regulation. Endocrinology. 1977;101(2):613-622.
96. Pizzi WJ, Barnhart JE, Fanslow DJ. Monosodium glutamate administration to the newborn reduces reproductive ability in female and male mice. Science. 1977;196(4288):452-454.
97. Tafelski TJ, Lamperti AA. The effects of a single injection of monosodium glutamate on the reproductive neuroendocrine axis of the female hamster. Biol Reprod. 1977;17(3):404-411.
98. Takasaki Y, Sekine S, Matsuzawa Y, Iwata S, Sasaoka M. Effects of parenteral and oral administration of monosodium L-glutamate (MSG) on somatic growth in rats. Toxicol Lett. 1979;4(5):327-343.
99. Matsuzawa Y, Yonetani S, Takasaki Y, Iwata S, Sekine S. Studies on reproductive endocrine function in rats treated with monosodium L-glutamate early in life. Toxicol Lett. 1979;4(5):359-371.
100. Matsuyama S, OkiY, Yokoki Y. Obesity induced by monosodium glutamate in mice. Natl Inst Anim Health Q(Tokyo). 1973;13(2):91-101.
101. Pizzi WJ, Barnhart JE. Effects of monosodium glutamate on somatic development, obesity and activity in the mouse. Pharmacol Biochem Behav. 1976;5(5):551-557.
102. Nikoletseas MM. Obesity in exercising, hypophagic rats treated with monosodium glutamate. Physiol Behav. 1977;19(6):767-773.
103. Redding TW, Schally AV. Effect of monosodium glutamate on the endocrine axis in rats. Fed Proc. 1970;29(2):378Abs (Abstract #755).
104. Holzwarth MA, Hurst EM. Manifestations of monosodium glutamate (MSG) induced lesions of the arcuate nucleus of the mouse. Anat Rec. 1974;178(2):378.
105. Trentini GP, Botticelli A, Botticelli CS. Effect of monosodium glutamate on the endocrine glands and on the reproductive function of the rat. Fertil Steril. 1974;25(6):478-483.
106. Lynch JF Jr, Lewis LM, Hove EL, Adkins JS. Effect of monosodium L-glutamate on development and reproduction in rats. Fed Proc. 1970;29(2):567 Abs (Abstract 1795).
107. Pradhan SN, Lynch JF Jr. Behavioral changes in adult rats treated with monosodium glutamate in the neonatal state. Arch Int Pharmacodyn Ther. 1972;197(2):301-304.
108. Iwata S, Ichimura M, Matsuzawa Y, Takasaki Y, Sasaoka M. Behavioral studies in rats treated with monosodium l-glutamate during the early stages of life. Toxicol Lett. 1979;4(5):345-357
109. Vorhees CV, Butcher RE, Brunner RL, Sobotka TJ. A developmental test batter for neurobehavioral toxicity in rats: a preliminary analysis using monosodium glutamate, calcium carrageenan, and hydroxyurea. Toxicol Appl Pharm. 1979;50(2):267-282.
110. Vogel JR, Nathan BA. Learned taste aversions induced by high doses of monosodium L-glutamate. Pharmacol Biochem Behav. 1975;3(5):935-937.
112. Berry HK, Butcher RE, Elliot LA, Brunner RL. The effect of monosodium glutamate on the early biochemical and behavioral development of the rat. Devl Psychobiol. 1974;7(2):165-173.
113. Weiss LR, Reilly JF, Williams J, Krop S. Effects of prolonged monosodium glutamate and other high salt diets on arterial pressure and learning ability in rats. Toxicol Appl Pharmacol. 1971;19(2):389.
114. Bhagavan HN, Coursin DB, Stewart CN. Monosodium glutamate induces convulsive disorders in rats. Nature. 1971;232(5308):275-276.
115. Johnston GAR. Convulsions induced in 10-day-old rats by intraperitoneal injection of monosodium glutamate and related excitant amino acids. Biochem Pharmacol. 1973;22(1):137-140.
116. Mushahwar IK, Koeppe RE. The toxicity of monosodium glutamate in young rats. Biochem Biophys Acta. 1971;244(2):318-321.
117. Nemeroff CB, Crisley FD. Lack of protection by pyridoxine or hydrazine pretreatment against monosodium glutamate induced seizures. Pharmacol Biochem Behav. 1975;3(5):927-929.
119. Wiechert P, Gollinitz G. Metabolic investigations of epileptic seizures: the activity of the glutamate decarboxylase prior to and during experimentally produced convulsions. J Neurochem. 1968;15(11):1265-1270. (Abstract)
120. Wiechert P, Herbst A. Provocation of cerebral seizures by derangement of the natural balance between glutamic acid and y-aminobutyric acid. J Neurochem. 1966;13(2):59-64.
121. Wiechert P, Gollnitz G. Metabolic investigations of epileptic seizures: investigations of glutamate metabolism in regions of the dog brain in preconvulsive states. J Neurochem. 1970;17(2):137-147.
122. Olney JW, Price MT. Neuroendocrine interactions of excitatory and inhibitory amino acids. Brain Res Bull. 1980;5:Suppl 2, 361-368.
123. Olney JW, Price MT. Excitotoxic amino acids as neuroendocrine probes. In: McGeer EG, Olney JW, McGeer PL eds. Kainic Acid as a Tool in Neurobiology New York: Raven Press; 1978.
124. Olney JW. Excitotoxic amino acids: research applications and safety implications. In: Filer LJ Jr, Garattini S, Kare MR, Reynolds WA, Wurtman RJ, eds. Glutamic Acid: Advances in Biochemistry and Physiology. New York: Raven Press; 1979:287-319.
125. Nemeroff CB. Monosodium glutamate-induced neurotoxicity: review of the literature and call for further research. In: Miller SA, ed. Nutrition & Behavior. Philadelphia: The Franklin Institute Press; 1981.

